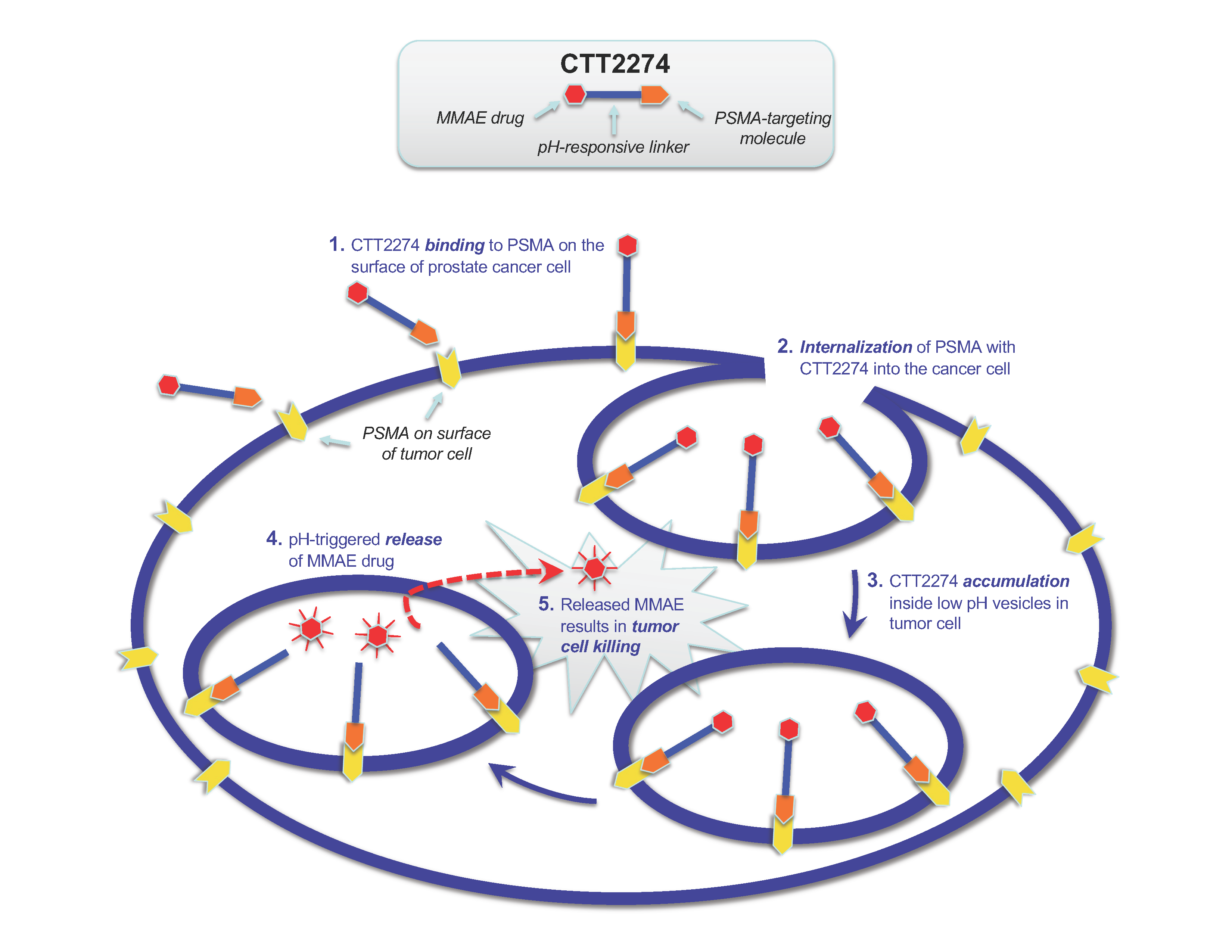
CTT2274 is a novel small molecule drug conjugate (SMDC) that targets prostate cancer cells using the same PSMA-targeting scaffold as CTT1057 and CTT1403. CTT2274 is designed to deliver the potent antimitotic payload, monomethyl auristatin E (MMAE) which is similar in action, but more potent than other approved taxanes used in metastatic castration resistant prostate cancer. CTT2274 uses a unique linker attached to the scaffold that acts as a prodrug. While MMAE is too toxic to be administered alone, this unique pH-sensitive linker of CTT2274 allows MMAE to be delivered only after CTT2274 is taken up by tumor cells, thus reducing off-target toxicities and undesirable side effects. Rapid drug release by the linker is ideal to quickly concentrate drug payload inside of the tumor cells. Using this unique delivery mechanism, CTT’s novel backbone, and PSMA-targeting, CTT2274, has the potential to both decrease toxicity of MMAE and result in substantial anti-tumor efficacy, offering a novel therapeutic for the treatment of advanced prostate cancer.
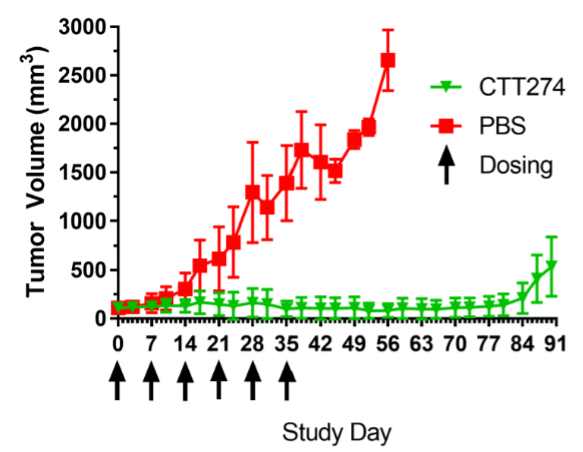
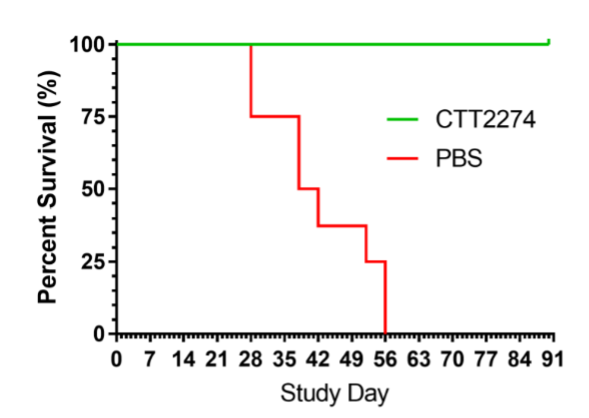
In a mouse efficacy study using a prostate tumor derived from a patient, once weekly dosing of CTT2274 resulted in sustained tumor suppression and significantly improved survival over mice treated with saline (PBS).
Continuing Development of CTT2274
In September 2024, CTT was awarded a $2.4M Fast-Track Phase I/II SBIR grant to support IND-enabling studies for CTT2274. CTT2274 is expected to enter first-in-human clinical trials in 2026.
